Rapid test for qualitative detection of: Bilharzia (Schistosomiasis) (25 Diagnostic tests) INDICATIONS FOR USE: The urine-CCA (Circulating Cathodic Antigen) cassette test is for the qualitative presumptive detection of an active Schistosoma infection, more specific S. mansoni, but also other species e.g. S. haematobium and S. japonicum. For endemic studies involving S.mansoni infections, a single CCA urine test demonstrates closely the true prevalence predicted by models based on multiple egg count determinations. Accuracy in urogenital schistosomiasis is variable, and also seems to differ between regions. In general, some medium to high level infections with S. haematobium can be diagnosed using the urine CCA strip. This test should be used for patients with clinical signs and symptoms that are consistent with a Bilharzia infection. CLINICAL SIGNIFICANCE: The urine-CCA cassette test is a fast and easy to perform methodology in the presumptive detection of Bilharzia in persons with clinical signs and symptoms consistent with an active bilharzia infection. A positive test result indicates an active infection. The test should only be used as one of the aids for the treating physician in assisting the diagnosis and treatment of an active infection. Positive results are presumptive and should take in account diagnostic regimes such as clinical history, clinical findings, microscopy based diagnosis on urine or stool, serological testing, biopsy and or histological examination of tissue. The test may be false negative in low levels of parasitic infection. The test result should be interpreted with caution during the premature developmental phases of a bilharzia infection, usually in the first 4-8 weeks after infection which may render a false negative result. Detection of antibodies against Bilharzia may further confirm the clinical suspicion. These antibodies however may persist for many years, even after successful treatment, making the diagnosis of a re-infection or the diagnosis of an unsuccessful treatment very cumbersome. Antibodies may also be absent in certain cases of chronic active infection. According to certain literature successful treatment after one standard recommended dose of chemotherapy is 65-85%. CCA rapidly declines after successful treatment and a positive test result usually becomes negative within 2-3 weeks after treatment. Re-infection may occur rapidly resulting in a positive test result within 6-10 weeks after initial successful chemotherapy. INTRODUCTION: Schistosomes are blood-dwelling flukes belonging to the class Trematoda, but differ from other trematodes having separate adult male and female parasites. Sexual reproduction happens in the definitive host (humans, cattle, etc), depending on the Bilharzia species and the asexual reproduction phase happens in the snail (intermediate host). Cercaria (released by specific snail species in the water) enters the human body through the skin. The young schistosomulum is most susceptible to immune damage. Employing certain evasion mechanisms, the worm becomes refractory, or even immunologically unrecognisable to certain aspects of the host defence mechanism. Adult parasites may survive for many years in the host, even up to 40 years. Approximately after 6 weeks post infection the adult worm-pairs start to lay eggs which penetrate the intestinal wall (S. mansoni, S. japonicum) or the bladder wall (S. haematobium) and will be passed out via the faeces or urine. A considerable proportion of eggs are not excreted but remain stuck in the tissue inducing granuloma formation with subsequent complications to the different organs affected. The gastrointestinal duct of a schistosome is a cul-de-sac. The parasite has to regurgitate at regular intervals the undigested particulate as well as "parasitic gut associated glycoproteins". One of the major antigens regurgitated by the parasites is CCA (Circulating Cathodic Antigen). Although Bilharzia eggs also release CCA antigen in minute quantities, the major source of CCA originates from live adult worms. DIAGNOSIS OF BILHARZIA: Laboratory diagnosis of Bilharzia is usually performed by microscopical detection of eggs in urine or stool or by immunological methods (antibody or antigen detection). Microscopic diagnosis is currently the most generally used method for detecting and confirmation of active Bilharzia. However, expert microscopic diagnosis is often not immediately available, and thus may delay the treatment in clinical suspected patients, or it may be unreliable or absent in remote areas. The sensitivity of microscopic examinations also depends on the severity of the infection. In low grade infections, the sensitivity of one microscopic examination may be as low as 20%. In clinically suspected cases up to 5 urine specimens (collected over midday), and or 5 stool specimens for microscopic examinations are recommended to increase the sensitivity of the tests. Due to immune modulation the infected host may show a separate IgG, IgM, IgA and IgE antibody response, or a combination of these isotypes. As much as 14% of patients may not respond with any antibody formation. Depending on the methodology used and the timing in the post infected host, the sensitivity of current antibody assays is not optimal. Some of the commonly used methodologies are based on detection of antibodies directed against the soluble egg antigen (SEA). Due to the retention of eggs and the constant secretion of SEA by the deposited eggs, antibodies may be elicited for an indefinite period after the primary infection, irrespective of successful treatment. The urine CCA cassette test detects the parasite antigen CCA which is present in all Schistosoma species, including animal species. The major portion of CCA released by the adult live parasite is secreted in the urine. A positive CCA test result on randomly collected midstream urine indicates an active Bilharzia infection. TEST PRINCIPLE: After applying the urine, the CCA antigen that may be present in the sample binds to the labelled monoclonal antibody immobilized on the sample membrane. The solution then runs over the strip where the antigen-antibody complex attaches to the monoclonal antibody immobilized at the test line. A pink-coloured line develops. The second line is a procedural control, which should always show up to make sure the test works correctly. SPECIMEN COLLECTION AND PREPARATION: Collect a urine sample in a urine cup. KIT COMPONENTS: Each kit contains the following components in sufficient quantities to perform the number of tests indicated on the package label: • 25 x test cassettes each individually packaged • 1 x instructions for use • 25 x pipettes ASSAY PROCEDURE: Note: Ensure all reagents are equilibrated to room temperature (20 - 25°C) before commencing the assay. Remove the test cassette and pipette from their pouches just prior to use. |
|
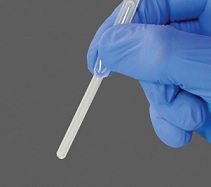 |
• Squeeze the pipette and insert the tip into the urine sample. • Allow the sample to fill up by gently releasing the pipette. |
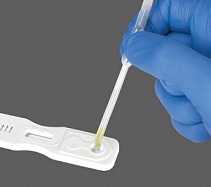 |
• Transfer 2 drops of urine to the circular well of the test
cassette by gently squeezing the pipette. (Each drop is equivalent to 45-50μL. When pipetting a total volume of 100μL is required.) • Allow the sample to absorb entirely into the specimen pad within the circular well. |
| • Read the result at 20 minutes. • Any results read after 25 minutes should be considered invalid and must be repeated. |
| PRECAUTIONS: |
|
| 1. | Keep storage boxes dry. |
| 2. | Do not reuse test cassettes. |
| 3. | Do not use test cassettes if foil pouch is punctured or damaged. |
| 4. | Never pipette by mouth or allow reagents or patient sample to come into contact with skin. |
| 5. | Optimal results will be obtained by strict adherence to this protocol. Reagents must be added carefully to maintain precision and accuracy. |
| 6. | Performing the assay outside the prescribed time and temperature ranges may produce invalid results. Assays not falling within the established time and temperature ranges must be repeated. |
| 7. | Care should be exercised to protect the reagents in this kit from contamination. Do not use if there is evidence of microbial contamination or precipitation. Biological contamination of containers, reagents or dispensing equipment can lead to false results. |
| 8. | Do not heat-inactivate samples. |
| 9. | All human urine products should be handled as potentially infectious material. |
| 10. | Waste disposal. Testing materials should be disposed of in accordance with local, state and/or federal regulations. |
INTERPRETATION OF RESULTS: POSITIVE |
|
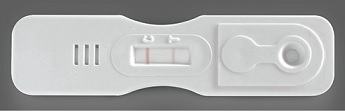 |
Control band turns pink. A band is present in the test T area. The test is positive for Bilharzia. |
| NEGATIVE |
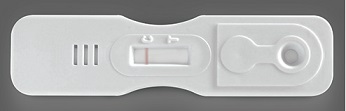 |
Control band turns pink. No test T band present. Demonstrates the test was performed correctly but no Bilharzia antigens were detected. |
| INVALID |
 |
No control line appears. The test is invalid and should be repeated. |
| |
 |
A test line with no control line. A pink control line must be present. The test is invalid and should be repeated. |
| CROSS-REACTIVITY: Urinary tract infections or haematuria may sometimes cause false positive results. QUALITY CONTROL: Quality Control (QC) requirements must be performed in conformance with local, state, and/or federal regulations or accreditation requirements and your laboratory's standard QC procedures. TEST LIMITATIONS: |
|
| 1. | The analysis of a single test sample should not be used as the sole criteria for diagnosis. |
| 2. | In early infections detectable levels of antigen may be absent. The worm load will also determine the sensitivity of the test. |
| 3. | In suspected clinical cases of Bilharzia, it should be kept in mind that the test may be false negative during the parasitic developing phase (first 6-7 weeks). |
| 4. | Re-testing or alternative testing methodologies should be
considered in such cases. Further investigation of negative results is therefore important. |
| 5. | Haematuria or pio-uria may cause a false positive test. |
| 6. | The final diagnosis should be based on the test result in conjunction with other clinical and or laboratory findings. |
| 7. | The continued presence or absence of CCA may be used to determine the failure or success of therapy. |
| 8. | CCA in urine decreases usually already the next day, and should become undetectable 2-3 weeks after successful treatment. |
STORAGE AND SHELF LIFE OF REAGENTS |
|
| 1. | Store kit between 4 °C and 40°C. Constant storage temperature must be maintained for the reagents to be stable until the expiry date of the kit. Refer to package label for expiry date. |
| 2. | Do not freeze kit components. |
| 3. | The test kit may be used until the expiry date marked on the package label. |
| 4. | Do not use reagents beyond the expiry date. |
STORAGE AND STABILITY OF URINE SAMPLES |
|
| 1. | Patient urine samples can be stored at 4°C for at least 7 days. |
| 2. | Patient urine samples can be stored at -20°C for at least 1 calendar year. |
PERFORMANCE EVALUATION DATA Sensitivity and specificity The sensitivity of the test varies with the intensity of the infection. Compared to the field gold standard for S. mansoni, microscopic egg determination, for intensities higher than 400 egg per gram of faeces, sensitivity is 100%. In low positive cases, the sensitivity can decrease to about 70%. However, also egg determination is highly variable and therefore shows decreased sensitivity, resulting in a comparable performance of both tests in field situations. For endemic studies, a single CCA urine test demonstrates closely the true prevalence predicted by models based on multiple egg count determinations. In negative or low endemic populations, the overall accuracy decreases. Lowest detectable limits In experimental animal models (baboons) it was determined that CCA can be detected in infections with about 50 worms and higher. The limit of detection by the CCA urine strip is at least comparable to the limit of detection by egg counts. Schistosoma species differentiation The highest concentrations of CCA are detected in S. mansoni infections, and therefore the test is particularly useful to diagnose intestinal schistosomiasis. Levels in urogenital schistosomiasis (S. haematobium) are variable, and also seem to differ between regions. In general there is a link between the sensitivity of the test and the intensity of the S. haematobium infection. Some medium to high level infection with S. haematobium can be diagnosed using the urine CCA strip. In general, the test lacks sensitivity for low level S. haematobium infections. DISCLAIMER The entire risk as to the performance of these tests and the use of the products is assumed by the purchaser. Rapid Medical Diagnostics shall not be liable for indirect, special or consequential damages of any kind resulting from the use of these products. |
|
BIBLIOGRAPHY References to published papers on the use of CCA urine strips. 1. Adriko, M., et al. 2014. Evaluation of circulating cathodic antigen (CCA) urine-cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda. Acta Trop. (2014). 2. Ayele, B., Erko, B., Legesse, M., Hailu, A. and Medhin, G., 2008. Evaluation of circulating cathodic antigen (CCA) strip for diagnosis of urinary schistosomiasis in Hassoba school children, Afar, Ethiopia. Parasite,15: 69-75. 3. Colley, D.G., Binder, S., Campbell, C., King C.H., Tchuem Tchuenté, L.A., N'Goran, E.K., Erko, B., Karanja, D.M., Kabatereine, N.B., van Lieshout, L., and Rathbun, S. 2013. A Five-Country Evaluation of a Point-of-Care Circulating Cathodic Antigen Urine Assay for the Prevalence of Schistosoma mansoni. Am. J. Trop. Med. Hyg., 88(3), 2013, pp. 426-432. 4. Coulibaly, J.T., N'Gbesso, Y.K., Knopp, S., N'Guessan, N.A., Silué, K.D., van Dam G.J., N'Goran, E.K. and Utzinger, J. 2013. Accuracy of Urine Circulating Cathodic Antigen Test for the Diagnosis of Schistosoma mansoni in Preschool-Aged Children before and after Treatment. PLoS Negl Trop Dis 7(3): e2109. doi:10.1371/journal.pntd.0002109. 5. Legesse, M. and Erko, B., 2008. Field-based evaluation of a reagent strip test for diagnosis of Schistosomiasis Mansoni by detecting circulating cathodic antigen (CCA) in urine in low endemic area in Ethiopia. Parasite, 15: 151-155. 6. Legesse, M. and Erko, B., 2007. Field-based evaluation of a reagent strip test for diagnosis of Schistosoma mansoni by detecting circulating cathodic antigen in urine before and after chemotherapy. Trans. R. Soc. Trop. Med. Hyg. 101:668-673. 7. Midzi, N., Butterworth, A.E., Mduluza, T., Munyati, S., Deelder, A.M., Van Dam, G.J., 2008.The use of circulating cathodic antigen (CCA) strips for the diagnosis of urinary schistosomiasis. Trans R Soc Trop Med Hyg (2008), doi:10.1016/j.trstmh.2008.08.018. 8. Odogwu S.Ee, Ramamurthy N.K., Kabatereine N.B., Kazibwe F., Tukahebwa E., Webster J.P., Fenwick A., Stothard J.R. 2006. Schistosoma mansoni in infants (aged < 3 years) along the Ugandan shoreline of Lake Victoria. Ann Trop Med Parasitol, 100:315- 326. 9. Pauline N. M. Mwinzi, Nupur Kittur, Elizabeth Ochola, Philip J. Cooper, Carl H. Campbell Jr., Charles H. King and Daniel G. Colley. 2015. Additional evaluation of the point-of-contact circulating cathodic antigen assay for Schistosoma mansoni infection. Frontiers in Public Health 2015. 10. Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, et al. ,2011. Evaluation of Urine CCA Assays for Detection of Schistosoma mansoni Infection in Western Kenya. PLoS Negl Trop Dis 5(1): e951. doi:10.1371/journal.pntd.0000951. 11. Sousa-Figueiredo J.C., Pleasant J., Day M., Betson M., Rollinson D., Montresor A., Kazibwe F., Kabater eine N.B., Stothard J.R. 2010. Treatment of intestinal schistosomiasis in Ugandan preschool children: best diagnosis, treatment efficacy and side-effects, and an extended praziquantel dosing pole. International Health, 2:103 - 113. 12. Sousa-Figueiredo, J.C., Betson, M., Kabatereine, N.B., Stothard, J.R. 2013. The Urine Circulating Cathodic Antigen (CCA) Dipstick: A Valid Substitute for Microscopy for Mapping and Point-Of-Care Diagnosis of Intestinal Schistosomiasis. PLoS Negl Trop Dis 7(1): e2008. doi:10.1371/journal. pntd.0002008. 13. Standley C.J., Lwambo N.J.S., Lange C.N., Kariuki H.C., Adriko M., Stothard J.R. 2010. Performance of circulating cathodic antigen (CCA) urine-dipsticks for rapid detection of intestinal schistosomiasis in schoolchildren from shoreline communities of Lake Victoria. Parasites & Vectors, 3:7. 14. Stothard, J.R., 2009. Improving control of African schistosomiasis: towards effective use of rapid diagnostic tests within an appropriate disease surveillance model. Trans R Soc Trop Med Hyg, 103:325 - 332. 15. Stothard, J.R., Kabatereine, N.B., Tukahebwa, E.M., Kazibwe, F., Rollinson, D,Mathieson, W., Webster, J.P. and Fenwick, A., 2006. Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis. Acta Trop. 97:219-228. 16. Stothard JR, Sousa-Figuereido JC, Betson M, Adriko M, Arinaitwe M, et al. ,2011. Schistosoma mansoni Infections in Young Children: When Are Schistosome Antigens in Urine, Eggs in Stool and Antibodies to Eggs First Detectable? PLoS Negl Trop Dis 5(1): e938. doi:10.1371/journal.pntd.0000938. 17. Tchuem Tchuenté, L-A., Kueté Fouodo, C.J., Kamwa Ngassam, R.I., Sumo, L., Dongmo Noumedem, C., Mérimé Kenfack C., Feussom Gipwe, N., Dankoni Nana, E., Stothard, J.R. and Rollinson, D. 2012. Evaluation of Circulating Cathodic Antigen (CCA) Urine-Tests for Diagnosis of Schistosoma mansoni Infection in Cameroon. PLoS Negl Trop Dis 6(7): e1758. doi:10.1371/journal.pntd.0001758. 18. Van Dam, G.J., Wichers, J. H., Falcao Ferreira, T. M., Ghati, D., van Amerongen, A. and Deelder, A. M., 2004. Diagnosis of Schistosomiasis by Reagent Strip Test for Detection of Circulating Cathodic Antigen. J. Clin. Microbiol. 42, No. 12, Dec. 2004, p. 5458–5461. 19. Van Lieshout, L., De Jonge, N., Mansour, M.M., Bassily, S., Krijger, F.W., Deelder, A.M., 1993. Circulating cathodic antigen levels in serum and urine of schistosomiasis patients before and after chemotherapy with praziquantel. Trans R Soc Trop Med Hyg, 87:311-2. Basic studies of CCA in urine 1. De Jonge, N., Kremsner, P.G., Krijger, F.W., Schommer, G., Fillié, Y.E., Kornelis, D., Van Zeyl, R.J, Van Dam, G.J., Feldmeier, H., Deelder, A.M. 1990. Detection of the schistosome circulating cathodic antigen by enzyme immunoassay using biotinylated monoclonal antibodies. Trans R Soc Trop Med Hyg, 84:815-8. 2. Disch, J., Garcia, M.M., Krijger, G.W., Amorim, M.N., Katz, N., Deelder, A.M., Gryseels, B., Rabello, A., 1997. Daily fluctuation of levels of circulating cathodic antigen in urine of children infected with Schistosoma mansoni in Brazil. Trans R Soc Trop Med Hyg, 91:222-5. 3. Polman, K., Diakhate, M.M., Engels, D., Nahimana, S., Van Dam, G.J., Falcão Ferreira, S.T., Deelder, A.M., Gryseels, B. 2000. Specificity of circulating antigen detection for schistosomiasis mansoni in Senegal and Burundi. Trop Med Int Health, 5:534. 4. Polman, K., Engels, D., Fathers, L., Deelder, A.M., Gryseels, B.1998. Day-to-day fluctuation of schistosome circulating antigen levels in serum and urine of humans infected with Schistosoma mansoni in Burundi. Am J Trop Med Hyg, 59:150-4. References to published papers on the use of CCA urine strips pre and post treatment and assessment of cure 1. Coulibaly, J.T., N'Gbesso, Y.K., Knopp, S., N'Guessan, N.A., Silué, K.D., et al. 2013. Accuracy of Urine Circulating Cathodic Antigen Test for the Diagnosis of Schistosoma mansoni in Preschool-Aged Children before and after Treatment. PLoS Negl Trop Dis 7(3): e2109. doi:10.1371/journal. pntd.0002109. 2. Koukounari, A., Donnely, C.A., Moustaki, I., Tukahebwa, E.M., Kabatereine, N.B., et al. 2013. A Latent Markov Modelling Approach to the Evaluation of Circulating Cathodic Antigen Strips for Schistosomiasis Diagnosis Preand Post-Praziquantel Treatment in Uganda. PLoS Comput Biol 9(12): e1003402. doi:10.1371/journal.pcbi.1003402. 3. Lamberton, P.H.L., Kabatereine, N.B., Oguttu, D.W., Fenwick, A., Webster, J.P. 2014. Sensitivity and Specificity of Multiple Kato-Katz Thick Smears and a Circulating Cathodic Antigen Test for Schistosoma mansoni Diagnosis Pre- and Postrepeated-Praziquantel Treatment. PLoS Negl Trop Dis 8(9): e3139. doi:10.1371/ journal.pntd.0003139. 4. Coulibaly, J.T., N'Gbesso, Y.K., Knopp, S., Keiser, J., N'Goran, E.K., Utzinger, J. 2012. Efficacy and Safety of Praziquantel in Preschool- Aged Children in an Area Co-Endemic for Schistosoma mansoni and S. haematobium. PLoS Negl Trop Dis 6(12): e1917. doi:10.1371/journal.pntd.0001917. 5. De Clercq, D., Sacko, M., Vercruysse, J., Vanden Bussche, V., Landouré, A., Diarra, A., Gryseels, B., Deelder, A. 1997. Assessment of cure by detection of circulating antigens in serum and urine, following schistosomiasis mass treatment in two villages of the Office du Niger, Mali. Acta Trop, 68:339-46. 6. Kremsner, P.G., Enyong, P., Krijger, F.W., De Jonge, N., Zotter, G.M., Thalhammer, F., Mühlschlegel, F., Bienzle, U., Feldmeier, H., Deelder, A.M. 1994. Circulating anodic and cathodic antigen in serum and urine from Schistosoma haematobium-infected Cameroonian children receiving praziquantel: a longitudinal study. Clin Infect Dis, 18:408-13. 7. Van Lieshout, L., De Jonge, N., Mansour, M.M, Bassily, S., Krijger, F.W., Deelder, A.M. 1993. Circulating cathodic antigen levels in serum and urine of schistosomiasis patients before and after chemotherapy with praziquantel. Trans R Soc Trop Med Hyg, 87:311-2.  www.ictdiagnostics.co.za, www.rapid-diagnostics.com ICT INTERNATIONAL,271 Goede Hoop Est, Village Lane, Noordhoek South Africa. Tel: +27 82 441 1922 Technical support: russell@ictdiagnostics.co.za Sales and orders: chantel@ictdiagnostics.co.za |